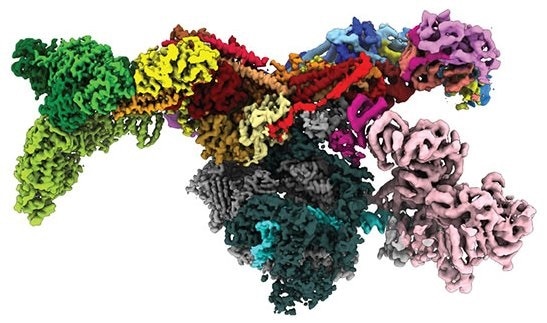
Numerous proteins bind to the DNA molecule in the cell nucleus to control the activity of specific genes. The TATA-box binding protein (TBP), which binds to a particular DNA sequence and acts as the initial signal for reading DNA, is one example. An enzyme known as Mot1 is used to “recycle” incorrectly bound TBP from the DNA. The Swi2/Snf2 remodelers, a broad family of molecular machines that utilise the ATP energy to disrupt protein-DNA interactions, includes this enzyme.
Researchers at LMU under the direction of Professor Karl-Peter Hopfner, Director of the Gene Center Munich, have now developed a comprehensive description of this previously poorly understood displacement mechanism. The researchers created several “snapshots” of the remodeling using cryogenic electron microscopy.
This process shows the varied workings of the Swi2/Snf2 remodeler family: all members have the same motor, but use it differently, whether it is to repackage DNA or – as in the case of Mot1 – to completely detach proteins from DNA. In the future, the researchers want to apply the acquired knowledge also to more complex Swi2/Snf2 molecular machines, which play a role in processes such as carcinogenesis or the development of neurons.